Article written by Bev Schechtman on May 11, 2022
Summary of our dramatic interaction with CDC's Opioid Rapid Response Program (ORRP)
Our other researcher, Carrie Judy, and I (Bev) initially heard about ORRP from a hearing transcript and a podcast interviewing a DEA agent.
ARPO’s efforts are not exclusively prosecutorial, however. Rapid response teams within the strike force work with HHS to ensure that persons physically dependent on or suffering from addiction to opioids are not getting turned away from the care they need just to seek out street dealers by directing these individuals to legitimate medical professionals and treatment resources.
We heard that this program was created to help find continuity of care for abandoned CPP's when a clinic was closed due to law enforcement. Great news, right? We had never heard of this program, and we thought maybe it was one answer to so much suffering. We reached out to them begging for their help. They were cordial, but told us that although it was their plan, they couldn't find doctors to take on abandoned CPP's other than Suboxone doctors for those with OUD. Doctors were just unwilling to see these patients. I spoke to the director of ORRP on the phone, and she asked me to be patient. She acknowledged that although the CDC Guidelines weren't laws, that many state laws were created based on them, and that was a problem. They were doing tabletop roleplay exercises at ORRP with states to practice what to do when clinics closed. So, hundreds of thousands of abandoned CPP's were suffering and some dying while the CDC was playing games. This was the only gov't funded program that we knew of that was supposed to help find care for abandoned CPP's, and they thought a good use of their funds was roleplay exercises? I hadn't heard from them for close to a year, so I sent an e-mail asking for another phone call in March of 2022.Things weren't so cordial this time.
As you'll see in the e-mail exchange below between ORRP and me from March, things started ok. I asked a follow-up question from our discussion last year about state laws. I asked since CDC is planning to remove hard thresholds, if had they planned to help people remove the 34 state laws that were based on the CDC Guidelines. That's when things took a dramatic turn.
My phone immediately rang, The ORRP director was on the other end and was angry and hostile. When I answered the phone, the ORRP director said in a hostile tone:
I wanted to help you, but we are under strict orders that if anyone even mentions the CDC Guidelines, we are to cease all communication immediately and forward all e-mails to our policy department.
Huh? Did I just enter the Twilight Zone? It certainly felt that way. She then proceeded to speak with a raised voice, hostile tone, and not let me get a word in. She cut me off, sighed loudly to show how annoyed she was, and spoke to me in quite a condescending way. Considering I'm from NJ, normally I can talk over anyone. But, I wasn't able to do that on this phone call. The phone call lasted 2 hours, and although it ended better than it began, it left me quite shaken. We have a summary of the phone call later in this article.
The theme of the phone call was that doctors are very bad, doing very bad things, and it's their fault their patients are abandoned because they obstruct all attempts to find doctors for their patients when their clinics are shut down. It seemed to me that ORRP was there to aid in investigations and not to help abandoned patients. One comment from this phone call was:
If doctors are breaking the law, they're not going to continue to prescribe.
Oh, and also CPP's aren't willing to try anything other than opioids, the ORRP director said. Don't worry, though. the CDC was working with local states to teach doctors how to properly treat pain. When I asked if I could suggest some doctors with balanced views, the ORRP director said she wasn't interested. She then asked me if I had ever heard of Dr. Don Stader. You know, the ER doctor who is with PROP and believes opioids just cause pain and don't treat it? The one who signed PROP's comment on the CDC Guideline's docket requesting for the hard thresholds to be added back in? Yes, that Dr. Stader. The ORRP director did tell me that they aren't there just to help abandoned CPP's due to law enforcement, but due to any reason such as a doctor dying or retiring, or a medical board investigation. She told me to send her any information regarding situations like this and if abandoned CPP's would e-mail her, she'd send them to the trusted contacts in each state for them to get help. When I asked for the list of trusted contacts in each state, the ORRP director said no, that wasn't possible, but if I wanted to, I could go over her head to Admiral Levine.
Claudia, who had been helping abandoned CPP's and those with OUD in Rhode Island after a doctor was shut down, e-mailed the ORRP director. She asked for help. I did what the ORRP director told me to do, and gathered information about closed clinics and abandoned CPP's. I gave out the ORRP e-mail address to abandoned CPP's like she asked me to do.
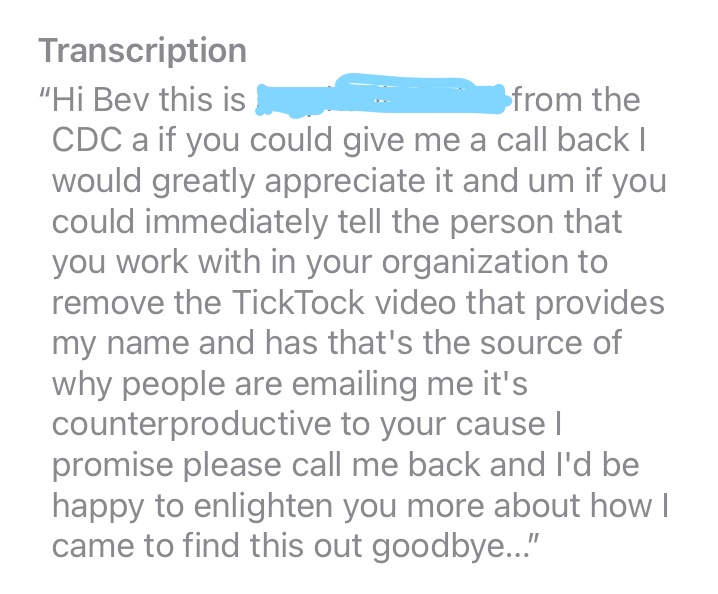
The ORRP director immediately sent me an e-mail telling me to not give out the e-mail address, that receiving so many e-mails was keeping her from doing her job. That was confusing since she had told me on the phone to give out the ORRP email address. She then left a voice mail on my phone demanding I have a Tik-Tok removed or else, well, or else it could hurt my cause. I thought that was odd since Isn't my cause the same as her cause? Isn't that her job? We included a screen shot of the voice mail below. She said:
If you could immediately tell the person you work with to remove the Tik-Tok video that provides my name...it's counterproductive to your cause, I promise.
Was that a threat? Hard to tell on the voice mail, but she did sound very angry. I responded asking for the link to the video since I couldn't find anything on TIkTok. There wasn't one, but the ORRP director never responded again. I found out that the ORRP director also called Claudia demanding the same thing, and Claudia told her she didn't know what she was talking about. She then hung up on Claudia after saying:.
You are a TERRIBLE human being.
What was she talking about, you ask? Well, one of the CPP's who emailed her had mentioned that she had found Claudia on TikTok. This was just background information as she had found Claudia several months prior to this email. The ORRP director jumped to conclusions thinking there was a TikTok about her. There wasn't. But, after all of that happened? Well, then there was social media content about this government agency employee and program. Claudia and I both felt quite intimidated by this situation.
We did try to contact the ORRP director's supervisor, Admiral Levine, but there was no response. The email is listed later on.
We include in this article screen shots of the message left by the ORRP director on my phone, all of the e-mails between us and ORRP, and quotes and a summary of the almost two-hour phone call between the ORRP director and me. The highlighted sections are where they blamed doctors and even patients. Those statements are very telling so I highlighted them so you don't miss them.
When we told people about ORRP, nobody had heard of it. Not doctors, health policy lawyers, Harm Reduction experts, or even local state agencies. Weird, right? A doctor who was indicted and is fighting for his freedom sent ORRP a FOIA (Freedom of Information Act) request on April 1, 2022. Since his practice was targeted by ARPO, supposedly ORRP would have helped his patients. He asked for all the information CDC had regarding ORRP. He wanted documents explaining the program and specifically any documents pertaining to his case. On May 9 he received a response stating they have zero documents at all about ORRP. How is that possible? What has the funding for this program been used for since 2018? We haven't been able to find one doctor or one patient this program has helped.
When I spoke to the doctor who submitted the FOIA, I searched to see if there was any new information online about this secret program. Low and behold a documentshowed up called "Proposed Opioid Rapid Response Patient Absorption Scoping Project." It was posted on April 15 and was a pre-request for proposal. It's by the CDC saying that although ORRP has existed since 2018, they haven't been able to find doctors for abandoned CPP's, and they couldn't figure out why. Huh, really? Anyway, they're offering $16.5 million to a consulting firm to give a proposal about researching this issue. There are some very important statements in this document, and we highly suggest you read them and even keep this document handy when you see your doctor. One statement is:
Clinicians not only need to be willing to accept a new patient; they also may need to initially prescribe a medication regimen that they otherwise might not endorse. This is because the alternative may go against CDC’s opioid prescribing guideline of avoiding forced or rapid tapering.
Wait, so now doctors are "violating the CDC Guidelines" if they "over-prescribe," and they're violating the CDC Guidelines if they don't prescribe? Got it. Great plan! At what point is it the fault of our government? The CDC? DEA? OIG? PROP? ARPO? The anti-opioid zealots who lied about this crisis to bring billions of dollars to our country though litigation? The government paid rats who have made millions as expert witnesses in litigation? We could tell the CDC for free that doctors don't take abandoned CPP's because they're afraid, and rightly so. Who will tell medical boards, payers, and the DEA to stand down if they do what this tells them to? Here are some bullet points from this document:
- ORRP is present in all 50 states.
- They can't find doctors to take these patients and they don't know why.
- They want a consulting firm to figure it out (budget of $16.5 million)
- They acknowledge that law enforcement actions cause abandoned patients, and since they can't find doctors they know CPP's sometimes go to the street and get illicit fentanyl laced pills or commit suicide.
- They said doctors need to take these patients and even if they are on "high doses" or on opioids and benzos, they need to keep them at their regimen, at least initially, or else they could be violating the CDC Guidelines. Did you catch that? So now if a doctor refuses to take abandoned CPP's, they may be held liable.
Here's our question: If this program has failed since 2018, why are they just deciding to look into this now? More than 4 years later? Is it because we've been posting about them? Is this damage control? Is the entire ORRP a front and a way for the CDC/DEA/OIG to show how they had a plan from the start for what to do with abandoned CPP's when a clinic is closed by law enforcement? Or is it a genuine program that just hasn't had enough funding? I know what I think, but you tell me. What do you think? Also, sure seems like since they're well aware that actions of DEA are causing harm to abandoned CPP's, they'd halt these raids to clinics. Yet, that's not happening. There were several press releases recently from ARPO bragging about their recent raids and indictments of 14 doctors. Let's say each doctor has 1,000 patients, just from that alone it could have left 14,000 abandoned vulnerable patients who are dependent on opioids for pain or OUD.
Keep reading for all the e-mails, a screen shot of the message left on my phone, and a summary of my phone calls (I took extensive notes so I have word for word quotes).
What is ORRP?
Opioid Rapid Response Program (ORRP) was supposedly created in 2018 as a result of ARPO (Appalachian Regional Prescription Opioid) strike force. ARPO is a strike force that is interagency between OIG, DEA, FBI, to shut down clinics or doctors who are "pill mills." Since they realized leaving patients without doctors would be harmful, they say they implemented the ORRP to work with State DOH to find continuity of care for patients (CPP's or someone w OUD). I first heard about this last year. I have spoken to many doctors who have been shut down by DEA, and none of them have heard of this program. None of the patients affected by these closings were helped by this program. Many of these patients are still abandoned. We even heard from a doctor that when he tried to help his patients find continuity of care, he was threatened by the DOJ.
The following are 3 brochures explaining the ORRP:
E-mails and phone calls between us (The Doctor Patient Forum) and ORRP
May 16, 2021 (Bev to ORRP)
So many clinics are closing or being closed. How do you help chronic pain patients so they continue to get their meds when a dr is raided or shut down? Is MAT the only option or do you help patients find another pain dr so they have continuity of care?
Thank you,
Bev Schechtman
May 17, 2021 (ORRP to Bev)
Hello Ms. Schechtman,
Thank you for your inquiry.
The current Opioid Rapid Response Program (ORRP) is designed to assist patients in need of referrals to pain management specialists as well as those in need of treatment and recovery support services. However, each state's capacity to facilitate referrals varies. Please refer to our updated website: https://www.cdc.gov/opioids/opioid-rapid-response-program.html and reach out to your state health department if you have questions about a specific recent closure in your state.
Sincerely,
The ORRP Team
May 17, 2021 (Bev to ORRP)
Thank you for your response. My question is a bit more specific than that. Every time a clinic or doctor's office is closed due to DEA or Med Board, it leaves medically abandoned patients. They have nowhere to go. The ER won't help, no other doctors will help, and sadly, many end up going to the streets to treat their pain. So, does your team actually help find doctors for these patients that will continue their opioid therapy? Or do they just taper or put on Suboxone?
May 17, 2021 (ORRP to Bev)
Hello Ms. Schechtman,
Thank you for your reply.
The ORRP does not provide any direct services for patients. If you would like to understand what the ORRP does and how it works, the CDC’s Opioid Rapid Response Program coordinator would be happy to talk over the phone. She can be reached at the contact below to schedule a call with you.
May 17, 2021 (Bev spoke to ORRP director)
Summary of this phone call:
- Although ORRP was designed to find continuity of care for abandoned CPP's as well as those with OUD, they are unable to find doctors to take abandoned CPP's.
- ORRP does table top role play exercises with local state agencies to practice what to do if a clinic is shut down.
- They are aware of the problem with abandoned CPP's but have no answers at this time.
- ORRP asked Bev to be patient because CDC was working on this problem.
- ORRP asked Bev to keep in touch.
May 18, 2021 (I emailed her regarding Lags closing and the abandoned CPP's) (Bev to ORRP)
I just wanted to send this to you. Anything you can do to help these people would be amazing.
https://abc30.com/lags-medical-center-closed/10654130/
May 18, 2021 (ORRP to Bev)
Hi Bev,
I briefly looked into it, and the abrupt closure of these clinics does not appear to be the result of a law enforcement action taken against them. I realize that doesn't change the situation for patients in need of pain management, but I think it's important to not assume that every closure that happens is the result of law enforcement going after prescribers. Something else was probably going on at this clinic or with the owners to make them abruptly close like that without any notice or concern for their patients. It's not something anyone wants to see happen, and unfortunately, it does put the patients at risk.
We continue to work at the federal, state, local, and tribal levels to seek solutions to these complex problems.
Thank you for being the voice of patients living with chronic pain management needs who also face stigma and barriers to care, just as people with substance use disorder do.
Kindly,
(name redacted)
June 13, 2021 (Bev to ORRP) (ORRP had requested a document listing state laws based on the CDC Guidelines, so I sent it to her)
I forgot to send this info to you
https://www.dovepress.com/an-examination-of-state-and-federal-opioid-analgesic-and-continuing-ed-peer-reviewed-fulltext-article-JPR
March 30, 2022 (Bev to ORRP)
Hi,
We spoke last year regarding the ORRP. You had mentioned that you were aware of the issue of abandoned CPP’s but couldn’t find doctors to take over prescribing. Has there been any more work in this area? The issue of abandoned CPP’s for a variety of reasons continues to grow as a direct result of the (misapplication) of CDC Guidelines. With Biden’s increased budget proposal to CDC for HR, I was wondering what the plan is to help these patients, which I’d suspect is now in the millions. U of M study last year showed about 40% of all doctors wouldn’t take a patient on opioids. I’ve contacted every gov agency and none have any plan to help. Since it’s supposed to fall under ORRP, I was wondering if we could plan a follow up call so I can get an update. Thank you!
Warm Regards,
Bev Schechtman
March 30, 2022 (ORRP to Bev)
Hi Bev,
I would be happy to speak with you about this issue, but yes, we are very much working on it. It is a major focus of our program. I personally coordinate with federal, state, and local agencies to facilitate care continuity for patients impacted by various disruptions in care. I am aware of the U of M study by Pooja Lagisetty as well as others noting upwards of 50% of patients taking long term opioid therapy will have trouble finding a clinician to accept them. We are working on it, it is not simple and it will not be solved overnight, but I believe we are making progress. It involves addressing a variety of issues including stigma, clinical training and support, unwarranted fears of increased scrutiny by law enforcement, and health system policies. If you have additional questions, please let me know and we can schedule a call. I do hope this brings you a bit of reassurance and hope, though I'm sure you wish (like I do) that it could be solved more immediately.
Thank you for reaching out.
March 30, 2022 (Bev to ORRP)
Sorry, I forgot to mention something in my email I just sent. I was reading an ORRP document from 2020 that stated that part of the program is to track patient outcomes through EHR w CMS. I was wondering where I can find that data for patient outcomes of both pain patients and those with OUD after a doctor or clinic is shut down. Thank you
Warm Regards,
Bev Schechtman
March 30, 2022 (ORRP to Bev)
Several possible program monitoring data source metrics were being considered in 2019 and 2020, but have not been used to date. DEA became a formal partner in September of 2021 and instituted an agency-wide protocol to partner with ORRP on all actions that could disrupt access for patients taking opioids. As a result we needed to devote resources to engaging in more state preparedness and response efforts, including clinical capacity building. Program outcome monitoring efforts have not been advanced as much as internal process monitoring to ensure timely notification by law enforcement and state risk mitigation response capability.
We are making a lot of progress on ORRP, but we have to be patient as we work across our many health systems to facilitate care continuity while also protecting patient data rights. Claims data is not publicly available. EHR data is private protected health information and was never considered as a data source for the federal program. Neither CDC nor state health officials working to mitigate risks to patients have access to patient-specific data for ORRP coordinated activities. So monitoring patient outcomes is challenging. In the future, hopefully we will have more resources to devote to an outcome evaluation.
From (Lead of ORRP)
March 30, 2022 (Bev to ORRP)
Ok, thank you. What is the process as far as addressing abandoned CPP’s? Are you still unable to find doctors to take over their care?
Warm Regards,
Bev Schechtman
March 30, 2022 (ORRP to Bev)
It's a very complex, layered process and each instance is different. It's not as simple as just identifying people to take the patients. It requires first identifying the patients and contacting them, coordinating with insurance, making sure the patient can access the new prescriber, ensuring workers comp patients get linked to a provider who takes WC patients, re-assessing patients.
Some disruptions affect different types of patients, some of whom are diverting medications which results in a disruption to the illicit drug supply and has its own set of risks, some of whom do have SUD in addition to chronic pain, some of whom are taking medications that are not effectively controlling their pain and are dangerous for them, and some of whom are taking medications appropriately and managing their pain effectively and safely. We actively work with states to identify clinics to accept displaced patients in their areas. FQHCs and Community Health Centers are a big focus for us, but they sometimes need training and support to handle complex pain management patients, and many of their clinicians are uncomfortable prescribing a lot of opioids. States issue alerts to clinicians encouraging them to accept patients and not to abandon them. We also coordinate with insurance plans to alert them when a provider who patients might be "locked into" have lost prescribing ability so they can unlock access to other providers, and we issue memos to payers telling them to facilitate care continuity for any of their impacted beneficiaries (because insurance companies are often the only ones with patient contact information and the legal authority to contact the patients directly). We provide academic detailing and rapid response training via tele ECHO sessions to clinicians and health systems inheriting displaced patients so they feel supported in providing care outside their comfort zone. States work with local telemedicine providers and mobile units to provide bridge care, and they staff call lines with care coordinators. Like I said, this is a complex, multi-faceted set of solutions because our health care system requires it. The process cannot be summed up succinctly. Basically, we do whatever we can to support states trying to find short-term as well as longer term care for affected patients. We don't have lists of patients that we can literally account for. That's private, protected health information. All licensed physicians are supposed to have emergency contingency plans for their patients. We have yet to encounter a physician who has a plan or is even helpful in finding new care for their patients. They have access to patient lists. CDC does not. State health departments do not. Law enforcement does not.
March 30, 2022 (Bev to ORRP)
Thank you for your answer. I would very much like to schedule a call. I must say, very little gives me hope as we hear from hundreds of CPP’s who are suicidal or are actively seeking pain care from black market. We are getting HR info to them to try to keep them alive, but doctors are too afraid to prescribe and we have a country full of abandoned patients. Often their only option is to falsely say they have OUD and go to a methadone clinic (often still not allowed) or go to the street and hope for the best. This isn’t just about abandoned CPP’s it’s also acute pain issues w them going to the street bc doctors won’t prescribe. Can I ask you if the CDC follows through w removing the MME and duration limits from updated guidelines is there a plan on how to remove the 34 state laws based on them? This is probably outside of what you’re responsible for, but just wondering if you might know.
Is there a day and time that works best for you?
Warm Regards,
Bev Schechtman
Within minutes of hitting send asking about the state laws, the ORRP lead called my phone. When I answered, she was irate. She opened the conversation by saying the following :
I wanted to help you, but we are under strict orders that if anyone even mentions the CDC Guidelines, we are to cease all communication immediately and forward all e-mails to our policy department.
As we mentioned earlier, It ended up being an almost two-hour phone call. Here are some quotes/bullet points from this call:
- "If a clinician is breaking the law, they cannot continue to prescribe." ~ORRP
- All agencies (DEA, law enforcement, regulatory agencies have communicated in black and white that those doctors who take on patients shouldn't be afraid. It's unwarranted fear. ~ORRP
- "Everyone is communicating, everyone is on board making sure we stop overdose deaths and stop abandoning patients."
- "It's easy to say they're afraid they'll be arrested, but when we dig a bit deeper, it is not that simple. They know that they're not going to get in trouble if they document what they're doing for a patient." ~ORRP
- "I'm not asking you to comment on whether or not they should be afraid to prescribe, I'm asking you if your program can help these patients...there are millions of abandoned pain patients." ~Bev
- "Who is helping abandoned pain patients?"~Bev
- "Give them my number and I will refer them to the OIG contact." ~ORRP
- "We have trusted contacts we work with in each state, and I would give them that number."
- "Can you give me the names of the trusted contacts in each state?" ~Bev
- "No, I can't do that." ~ORRP
- "Have the patients email me, that's literally what we are here for." ~ORRP (With an attitude)
- "Can you tell me why you're coming across with such an attitude when I'm begging you for help because our people are dying." ~Bev
- When patients call we give them things they can do, and one of those things is the suicide hotline because they're in so much pain they suicidal ~ORRP
- After trying to speak repeatedly, Bev said "Can you please let me speak. I've been listening to you and you don't let me finish a sentence." ~Bev
- "I've been responding to your emails all day." ~ORRP
- "You may think it's only a handful of people, but we have million people every day in every state who end up going to the street because there is nobody to help them, and now we have to give them HR resources to try to keep them alive."
- "I do realize this." `ORRP
- "Then why are you acting annoyed like I'm taking you away from your job, when this is your job. NOBODY has been willing to help abandoned pain patients. NOBODY" ~Bev
- Bev asked why the DOH doesn't offer these resources when we've called begging for help.
- ORRP asked where the primary care physicians are and why they don't prescribe
- ORRP said part of their job is to tell primary care physicians they are allowed to prescribe
- "We now have patients forced down to 50 MME because of the new guidelines." ~Bev
- She mentions the revised guidelines allows doctors to prescribe. ~ORRP
- "The guidelines need to be read in their entirely, and we can't know for sure whether doctors will misinterpret, misuse, or misapply the guidelines." ~ORRP
- About the guidelines, ORRP said the CDC had to provide evidence behind certain things.
- Bev told her there is no evidence behind MME.
- "There are laws based on those thresholds, though." ~Bev
- "That's not on the CDC, call the DOJ." ~ORRP
- "There are laws based on them, data analytic programs, Where was the CDC to help us fight? If that was as misapplication, who is helping us fight?" ~Bev
- "We have millions of patients who can't find doctors, and the only ones willing to help these people are drug dealers." ~Bev
- "Not one state has helped when we've asked for help with abandoned CPP's" `Bev
- "I'm proud of our progress, and this is not an easy job." ~ORRP
- "There is a lot of breakthroughs in pain management using buprenorphine."
- "There is no more long-term evidence on bupe for pain than there is regular opioids. We actually have more evidence on regular opioids for pain. It is just a schedule 3, so doctors feel more comfortable prescribing." ~Bev
- "The reality of our health care system is they are not doing continue to ramp up long-term opioid prescribing for chronic pain. Those days have largely ended." ~ORRP
- "Since this is where we are, we can't continue to abandon legacy opioid patients." ~ORRP
- "This is after the fact. We are abandoned." ~Bev
- "Doctors are afraid for a reason." ~Bev
- "We got DEA to issue a statement to say they won't be under increased scrutiny for prescribing inherited patients, just like they have never been under increased scrutiny." ~ORRP
- "There are kids going without parents. There are people who were fully functional with a family and a job and they kill themselves because they can't deal with their pain." ~Bev
- "Who is telling the DEA/OIG to remove their red flags based on 90 MME?" ~Bev
- "They don't have those red flags." ~ORRP
- "OIG is looking at their algorithms because they know they're faulty, but their algorithms don't lead to an arrest." ~ORRP
- "That's not true. These cases against pharmacies absolutely do list red flags from algorithms and they use the CDC Guideline limits to go after doctors." ~Bev
- "I have seen that happen." ~ORRP
March 31, 2022 (ORRP to Bev)
Bev, I'm sorry we got disconnected yesterday afternoon. It was probably when my phone switched from Wifi to cellular as I left the house to pick up my kids. I just wanted to thank you for the conversation. I hear you and I understand the issues. We are trying to find solutions to help the masses.
I'm attaching a letter that was sent to all providers by the California DPH following the LAGs clinic closures. We have shared this letter with other states and know of several working on their own similar letters to clinicians.
I've already received outreach from an advocate in RI requesting assistance for palliative pain patients, and I will be notifying our state contacts of the need and offering our technical support to them. RI is one state where we have not received any notifications of disruptions or had any interaction with the response coordinators, so patience is needed as we work to support the state.
Kindly,
ORRP Lead
March 31, 2022 (Bev to ORRP)
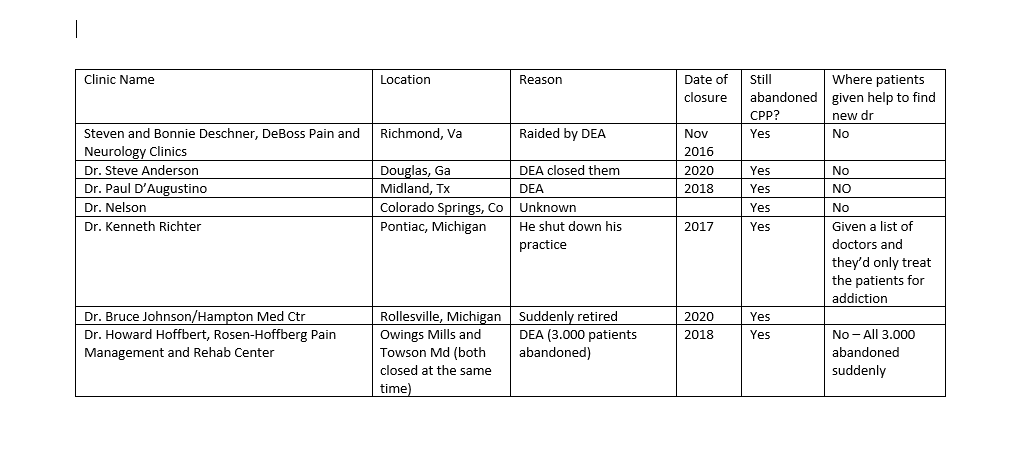
Thank you for the letter. It is encouraging to know that you are aware of just how dire the situation is. I am gathering information to send you all in one document so you don't get bombarded with too many emails at once. I am creating a chart with information of abandoned CPP's due to 1. The dr retiring, 2. the dr moving 3. the dr being investigated by the Med Board or 4. The doctor being investigated by DEA. I will be including name of dr or clinic, location, date of closure, if there are still abandoned CPP's or PWSUD needing continuity of care, and if any of the patients were offered any help.
If I understood you correctly, you said when a doctor is closed for whatever reason, you have no way to access a list of patients to contact to offer help. Is there any way that when this happens, the doctor can be required to contact every patient and give a number for them to call and/or maybe post a letter with the same info on the door of the clinic and website? Or can ORRP post a letter on the door so patients know who to call? In just a few minutes I've already heard from 5 situations like this and none of them have been offered help.
I also will be adding to our Non-Profit's website steps to take if abandoned due to closure of clinic. If there is a situation like this, which email address would you like them to use to contact you? In addition to contacting you to inform you of the situation, based on what you said yesterday, I will tell them to call their DOH and ask for a patient advocate in overdose prevention to help them find continuity of care. If there is anything else they can do to access the resource ORRP provides, please let me know.
March 31, 2022 (ORRP to Bev)
I will take a look at your list when you share it and determine what we might be able to do with it. Unfortunately, we are building this program out at the Federal level and hoping that state-led licensing actions follow our lead once states improve their overall understanding of the risks and build out response protocols. But we include state regulatory agencies on our preparedness training exercises with states so they can understand how important timely communication and coordination is when a clinician loses their ability to prescribe.
Please do not list my contact information for individual patients to call for individualized needs. We do not provide direct patient services. They need to contact state and local health resources as you noted below. Patients are generally directed to do the following. Yes, posting flyers on doors of clinics is the minimum state and local HDs do. Most often, the flyers are removed by the physicians. We have literally had HDs posting them repeatedly every day only to have them removed.
- If the office has an office manager, call them and ask them for assistance
- Call their primary care provider for assistance
- Call their insurance provider for assistance finding a provider
- Go to the emergency room for a bridge prescription
- Call a crisis hotline
- Do not turn to the illicit market
I know you want a better option, but that is the best we have right now. One other avenue to call would be state health departments’ Division of Consumer Protections for assistance finding a clinician who is able to care for patients with severe chronic pain.
No one has the authority or ability to force the doctors to do anything for their patients, but we have had some success in our coordination with law enforcement by asking them to speak with cooperative office staff to assist patients as well as instructing clinicians to assist patients with finding new providers to take over their care. Sometimes, they cooperate and want to help patients, but other times they do not. As a condition of their licensing, physicians are supposed to have an emergency care continuity plan for all patients. We have only had a few experiences where this is in fact the case.
March 31, 2022 (Bev to ORRP)
I told them not to contact you for individual abandoned patients who were dismissed for whatever reason. The vast majority of these abandoned CPP’s due to clinics closing have already contacted their local med board and dept of health, and have called every possible dr to find continuity of care, so I told these people to email ORRP with this type of situation. I’m not sending you info on individual patients and I’m not including any patient’s names w the info of closed clinics.
So you’re saying every time a clinic is shut down, the state DOH posts a flyer w info on what to do but the doctors remove them? Why would they remove them if it’s giving patients info on what steps to take?
March 31, 2022 (ORRP to Bev)
Thank you!
Not every time a clinic is closed down. Every time we work on an ORRP-coordinated action, which is every time we are notified of a potential disruption in patient access to controlled substances as a result of a federal action – taken by DEA or HHS OIG, we encourage the creation and posting of flyers like the one attached, with information tailored to the specific situation and suspected patient needs. When health officials are not permitted on site at the time of the action, we provide the flyers directly to the agents onsite who provide them to patients and leave them with office staff and even post them on doors before they leave the scene. Ideally we get state or local health professionals on the scene, but it is not always possible for safety, logistical, or other reasons.
We do not have any mechanism by which states notify us when state-led actions occur. Some states coordinate internally (KY is one of them). DEA has been notifying us when they get a DEA surrender subsequent to a state controlled substance license revocation or surrender, but that is sometimes months after the state action occurred. For example, the case in RI I learned about this morning was a state license voluntary surrender after a medial board order. It happened a couple of weeks ago, so it makes sense patients are now contacting advocacy groups. DEA might not even know about that disruption yet. We have a call with RI health officials first thing tomorrow morning to talk through patient needs.
They take them down probably because they don’t want a sign that highlights a problem and patients’ needs. I realize the docs may come across as victims to their patients when they are subjects of investigations. We see another side. The vast majority of the doctors we encounter through DEA and HHS OIG actions are generally obstructive and at best non-responsive to health officials’ efforts to facilitate care coordination and referrals.
April 1, 2022 (ORRP to Bev) (While on our phone call, she had told Bev to give out the ORRP email address for patients to contact her)
Bev, This is the second email from a patient that I have received since speaking with you. Someone is providing my direct email. I will not be able to respond to all requests like this, and hope they have appropriate expectations. A lack of response could lead to even more feelings of abandonment, but I cannot do my job if I keep receiving many of these emails.
CDC also does not coordinate treatment services. This is highly counterproductive.
April 1, 2022 (Bev to ORRP)
I gave the email per what you said was ORRP@cdc.gov and said this is only if a clinic closed and not for individual situations. I specifically stated not to email you for individual situations. I didn’t give out your personal email. If you responded to them on it maybe they gave it out. I can send you a screen shot of my post. I 💯 did not post your email address
April 1, 2022 (Bev to ORRP) This was in response to being told I should not give her email address out
Here is a screen shot of my post. I’m not sure how anyone got your other email address

April 1, 2022 (Bev to ORRP)
I’m working on the info for you of clinics that were closed, but we were called today by a patient in Mississippi who is at a clinic and they were told it’s closing in 2 months, and no help is being given of where they can go. There are no PM centers for hundreds of miles, according to him. They weren’t given a Reason for the clinic closing, so I don’t know if it’s to do w DEA, Med Board, or a different reason. But if they don’t get some kind of help, there will be more deaths, especially those being treated for OUD there.
April 1, 2022 (ORRP to Bev)
Bev, methadone clinics, like all businesses close for all kinds of reasons. If they have a 2 month warning on the door, it is highly unlikely it is due to a law enforcement action. Also, CDC does not handle treatment authority. They need to call their SAMHSA state opioid treatment authority (SOTA). Every state has one. https://www.samhsa.gov/medication-assisted-treatment/sota. Methadone clinics are highly regulated. Again, I cannot help with every type of disruption for individual patients. There are resources for them and CDC is not the right place to direct these requests.
April 1, 2022 (ORRP to Bev...she provided contradictory info from when we spoke on the phone)
Sounds like the same exact scenario from (name redacted) in MS who says he takes Methadone, so not sure if that's just a coincidence. If you know of a pain clinic closing for any reason in a state, you can contact the state health department and ask that they look into it and consider implementing an Opioid Rapid Response Protocol to mitigate risks for the affected patients, if possible. You can provide the link to the ORRP CDC Website. I would present it as a service -- you are letting them know about an upcoming clinic closure that could impact patients. They may or may not have the resources to respond to every closure, and they may or may not be able to confirm what you tell them based on resources and competing demands. I know that can be hard to hear, but the reality is public health has major workforce shortages and health departments cannot always activate a response or move at the speed you wish they would.
April 1, 2022 (Bev to ORRP)
It is the same patient but he takes methadone for pain from a pain clinic. It isn’t just an OUD med.
April 1, 2022
I am aware. The closure of a pain clinic in a remote area is not unheard of and the challenges are severely exacerbated by a lack of pain management specialists. Patients are encouraged to get referral assistance from the current clinician, their insurance provider, or their primary care physician.
Unfortunately, our program is not able to assist with individual patients' needs.
April 1, 2022 (Bev to ORRP)
Ok, maybe I miscommunicated. I’m not asking you to help the individual patients. You told me you wanted to be told about clinics closing due to any reason and ORRP could try to assist. You mentioned helping a Tx prescriber who decided to retire. So then your program is only for OIG closures?
April 1. 2022 (ORRP to Bev)
No, not at all, but I would need to at least know of the name and location of the clinic and the physician's full name, and I would prefer if the closure could be verified first.
DEA and OIG already notify us of their actions. So you don't have to notify us about those.
If you know of a closure happening at a specific clinic with a specific provider, we will notify the state and ask them to look into it, assess patient risk and let us now if they need help with risk mitigation. That's all I can do. With DEA and OIG, we work closely with them to identify risks based on information they have about the case.
Your post is inaccurate for several reasons. 1 - we support states in their efforts to mitigate risks for patients using a variety of strategies, but CDC does not facilitate care continuity directly. Also, it is not always the health department that "assists" patients. The public health and behavioral health agencies collaborate to coordinate a response intended to mitigate risks and facilitate care continuity -- they may leverage various community, local, or state agencies and organizations to assist. They also usually do not have access to specific patient information. Do you see how your messaging that states "...if you haven't been offered help by your Dept of Health" misrepresents the power and data access that a health department has and could lead to even greater anger and frustration by patients? Patients are understandably afraid and angry and frustrated, and honestly, the person most to blame is usually their doctor. But I am concerned that making them feel that some specific agency isn't doing their job to help them only adds fuel to an already heated situation. Clinics trying to help displaced pain patients have been threatened with violence and literally had to close because of repeated threats from patientsfor fear of losing more health care workers from the overburdened health care workforce. These are the same clinics we are trying to convince to take displaced pain patients in. So please understand, words and messaging matter.
Providers firing their patients and closing abruptly can be complex especially, for instance, when it is a concierge doc, like in RI. No patient records, no files, no cooperation. There may be little the state can really do to help other than alerting health care providers and encouraging them to take the patients in and re-assess their treatment needs. That said, no good provider is going to accept a patient's word that they have a diagnosis and prescribe controlled substances blindly. So we are talking about the need to schedule new intake visits, which take time. And yes, I know the patients don't have time and can experience withdrawal and untreated pain in the meantime. Like I said, this is not easy.
April 1, 2022
ORRP lead left the following message on my phone:
April 1, 2022 (Bev to ORRP)
I don’t know anything about a TIk Tok. I sent screen shots of what I posted.
April 2, 2022 (Bev to ORRP) (Bev requesting info lead of ORRP said she would send...Bev still hasn't heard back from her)
Please send me the following info:
1. Pa patient advocacy program you said you’d send me
2. Contact info for Admiral Levine since you suggested I contact her to get the list of trusted contacts in each state.
Also, I don’t know anything about a tik tok. What do you mean by if we don’t immediately remove it you promise it will be counterproductive to our cause? Not sure what you mean by “our cause” or by promising it will be counterproductive. If you mean finding help for abandoned CPP’s then is that not your cause also?
April 4, 2022 (Bev to ORRP) (There wasn't a Tik Tok, and she never responded)
If you send me the link of the video you’re referring to it would be helpful because I truly have no idea what you’re referring to. I don’t know anything about a tik tok and I sent you the screenshot of the post I created w the ORRP email address, which is what you told me to give to abandoned CPP’s.
April 5, 2022 (Bev and Claudia sent an email requesting a meeting with Admiral Rachel Levine. No response was given)
Admiral Rachel Levine,
Our names are Bev Schechtman and Claudia Merandi. We run a National Non Profit called The Doctor Patient Forum. We both have Crohn's Disease and have been advocating on behalf of chronic pain and illness patients since 2017.
Due to the overdose crisis there has been tremendous crackdown on prescription opioids. Due to a variety of reasons our country now has hundreds of thousands, if not millions of abandoned chronic pain and illness patients. These patients aren't just left without a doctor to prescribe, they're left without a doctor at all, and are unable to find a clinician to accept them as patients. They are not welcome in the ER and when we've contacted state medical boards or HHS they don't have any program to help.
Some of the reasons this occurs are a clinic is shut down by HHS OIG-DEA due to being investigated, a clinic is investigated by their medical board, a doctor passes away, a doctor retires, a doctor dismisses all of their chronic pain patients due to too much red tape, or even individual patients who are dismissed from a practice for things like a "failed" UDT, a high-risk score such as NarxCare, or even for just requesting an increase of medication due to undertreated pain.
We have reached out to every Government agency we can think of to request help for these abandoned patients. We get daily calls, e-mails, messages from desperate abandoned patients who often say the same thing "it's either suicide or the streets." Due to this we have added Harm Reduction information to our website to hopefully prevent them from dying from pills laced with illicit fentanyl. For the past four years we have volunteered our time do everything we can to place abandoned patients with providers, but it is getting increasingly difficult to find providers to take these patients due to fear of law enforcement.
We respectfully request a meeting with you, Admiral Levine. We have read some concerns you've had in the past about abandoned patients and have high hopes that you will accept a meeting with us so we can tell you our ideas on how to help address this crisis in our country. We can't just keep ignoring the fact that there are probably millions of abandoned patients with nowhere to turn but the streets. This problem is greatly adding to the overdose crisis, and it is something tangible we can do to prevent these needless deaths. We look forward to hearing from you.
April 6, 2022 (Bev to ORRP) (Per our conversation I told her I'd send her details from closed clinics where there were abandoned CPP's)
Here is the first batch. I have probably about 25 more that I'm organizing in a chart. (I sent a document with closed clinic info, which is what she requested)
April 20, 2022 (Bev to ORRP, I attached a pic of this announcement)
I would still appreciate the information you were supposed to send about the Pennsylvania contacts. Also, anything you can do for these patients so they don’t end up on the streets and dying would be greatly appreciated.

May 3, 2022 From Claudia to Stephanie
Stephanie,
Are you still with the CDC? We are still awaiting a response, as well as a meeting with Rachel Levine.
Bev has reached out to several governmental agencies as well as a contractor for the CDC. None are willing to help abandoned patients locate doctors who are willing to treat them.
Would you please respond to this email?
Have a great day
--
Claudia A. Merandi
FOIA Request From a Dr. to CDC about ORRP
A doctor who is currently fighting to stay out of prison sent ORRP a FOIA request for all documents pertaining to ORRP.
Not surprisingly, CDC had nothing to show. Here is their response.
Pre-Request For Proposal from CDC for a Company to Study Why Doctors Won't "Absorb" Abandoned CPP's
Their stated budget is $16.5 million. So, They don't have money to create a program that actually can help CPP's. But, they have millions to give to a company to tell them what everyone knows already, which is DOCTOR'S ARE TOO AFRAID OF DEA, MEDICAL BOARDS, AND PAYERS to take on abandoned CPP's. Do you blame them? I certainly don't.
PDF of this request
Link to this request
Some quotes from this document:
- "Opioid Rapid Response Program (ORRP) is an interagency, coordinated federal effort to mitigate drug overdose and other risks among patients impacted by law enforcement actions that disrupt access to prescription opioids or medication assisted treatment/medication for opioid use disorder (MAT/MOUD). ORRP supports care continuity and risk reduction for patients by coordinating federal law enforcement actions and public health risk mitigation."
- "ORRP (then known as Opioid Rapid Response Teams) was founded to help state and local authorities ensure that patients dependent on pain medications, who lost access to a prescriber due to ARPO efforts, were directed to reputable professionals and addiction treatment providers."
- "A challenge the ORRP staff observed when working with state health officials was their difficulty identifying clinicians willing and able to absorb displaced patients, particularly those patients who had been receiving high dose long-term opioid therapy or combinations of medications that are contraindicated such as benzodiazepines and opioids."
- "When patients cannot find access to a new clinician, they may be at risk of seeking relief from withdrawal or pain in the illicit market. There they are at risk of encountering dangerous counterfeit pills that could contain fentanyl. In addition, patients with chronic pain are also at risk of suicide."
- "clinicians not only need to be willing to accept a new patient; they also may need to initially prescribe a medication regimen that they otherwise might not endorse. This is because the alternative may go against CDC’s opioid prescribing guideline of avoiding forced or rapid tapering."
- "Because a primary goal of ORRP is to facilitate care continuity for patients impacted by disrupted access to opioid prescriptions, CDC needs to better understand ways to support states and encourage adoption of displaced patients on long-term opioid therapy (also known as “legacy” opioid patients) by clinicians and health systems."
Final thoughts
I never thought an employee at a government agency would call to intimidate a patient. Claudia and I felt quite intimidated. Our goal in exposing the program isn't to shame anyone. We were hoping we could use this information to get help to the millions of medically abandoned CPP's. Unfortunately, that hasn't happened. Often, as you know, it's not only that patients can't find doctors to prescribe what they've been getting, they often can't find doctors at all. The fact that the director of ORRP acknowledged this fact stating she knows they are suicidal or going to the streets, made me more disgusted with the entire issue. They know we are dying. Instead of putting that $16.5 toward helping find care for abandoned CPP's, they're going to research why doctors won't take patients. It's not that complicated. In order for doctors to take patients, our government agencies and medical boards will have to assure the doctors they are safe. They won't do that, even though ORRP said they've made that clear to doctors that they're safe to prescribe. We are well aware, as we told ORRP, that out of desperation for pain relief, some patients obtain their medication from the street. If you are aware of anyone doing this, please share our Harm Reduction information. It contains info on naloxone illicit fentanyl testing strips, Kratom, and more.
That document CDC posted on April 15 is helpful to us. If you're a CPP who was abandoned and you're told you must taper, please show them that document. Also, if you are a doctor and you inherited abandoned CPP's continuing their medication regimen, and have been sent threatening letters from payers, or have been investigated by your medical board, please show them the document. It clearly states you must, at least initially, continue their medication regimen, even if it is opioids and a benzo.
The phone call I had with ORRP director admitted OIG algorithms are flawed and they know it. I'm hoping this can help doctors who have been targeted by these algorithms.
Please don't send threatening or nasty letters to the CDC regarding ORRP on our behalf. We understand your anger, we are angry also, but it won't help us to accomplish our goal of finding continuity of care for abandoned CPP's.
If you've been medically abandoned or force tapered, please share your story with us using our Contact Us form. Please read through our website. We have a lot of valuable information like Advocacy Tools and Debunking Lies.